Solutions
Products
-

Primary mobile crushing plant
-

Independent operating combined mobile crushing station
-

Mobile secondary crushing plant
-

Fine crushing and screening mobile station
-

Fine crushing & washing mobile station
-

Three combinations mobile crushing plant
-

Four combinations mobile crushing plant
-

HGT gyratory crusher
-

C6X series jaw crusher
-

JC series jaw crusher
-

Jaw crusher
-

HJ series jaw crusher
-

CI5X series impact crusher
-

Primary impact crusher
-

Secondary impact crusher
-

Impact crusher
-

HPT series hydraulic cone crusher
-

HST hydraulic cone crusher
-
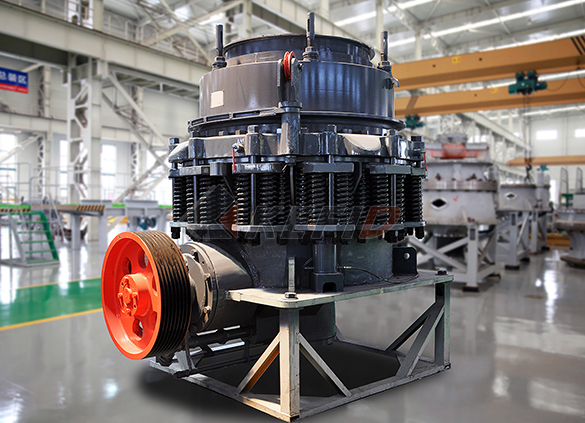
CS cone crusher
-

VSI6S vertical shaft impact crusher
-
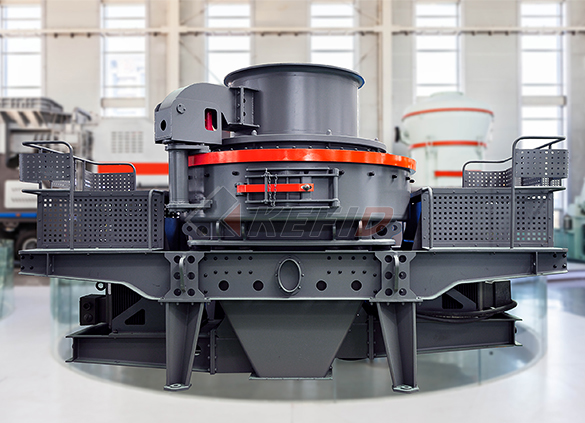
Deep rotor vsi crusher
-
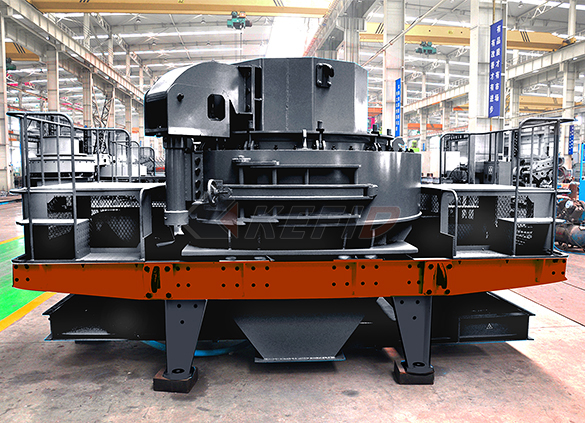
B series vsi crusher
-

Vertical grinding mill
-

Ultra fine vertical grinding mill
-

MTW european grinding mill
-

MB5X158 pendulum suspension grinding mill
-

Trapezium mill
-

T130X super-fine grinding mill
-

Micro powder mill
-

European hammer mill
-

Raymond mill
-
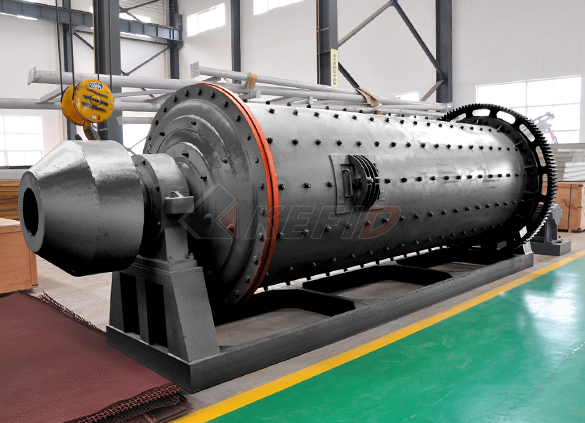
Ball mill
-
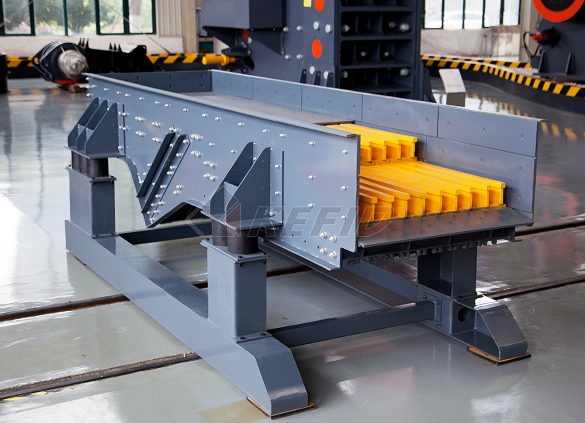
GF series feeder
-

FH heavy vibrating feeder
-

TSW series vibrating feeder
-

Vibrating feeder
-

Vibrating screen
-

S5X vibrating screen
-

Belt conveyor
-

Wheel sand washing machine
-

Screw sand washing machine
-

Rod mill
-

Dryer
-

Rotary kiln
-

Wet magnetic separator
-

High gradient magnetic separator
-

Dry magnetic separator
-

Flotation machine
-

Electromagnetic vibrating feeder
-
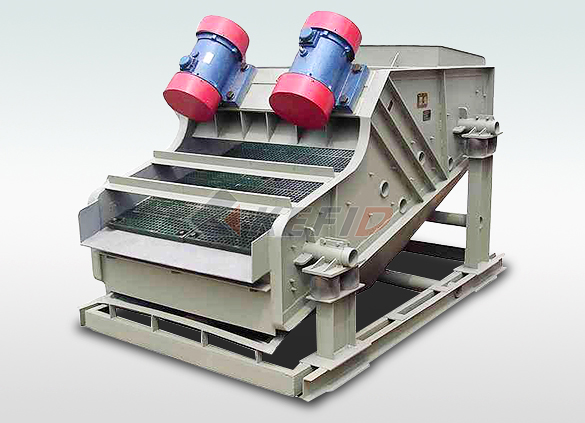
High frequency screen

Distinguishing between Crystalline and Amorphous
The atoms in crystalline solid matter are arranged in regular, repeatingpatterns All other types of solid matter are amorphous or without a regular atomic arrangement Metals and The graphical representation of the cooling characteristics of an amorphous solid gives a smooth curve, while that of a crystalline solid has two break points along the curve These break points indicate the beginning and end of the crystallization process, during which, the temperature remains constantCrystalline Vs Amorphous Solids What's the Difference Between Crystalline and Amorphous There are three states of matter namely solids, liquids and gases Solids have two states namely amorphous and crystalline form The particles are arranged with a definite or indefinite geometryDifference Between Crystalline and Amorphous in The fundamental difference between crystalline body and an amorphous substance lies in the fact that while in crystalline body, there exists a regular arrangement of the atoms in space, amorphous substance is characteristically devoid of such regular arrangement, amorphous substance the atoms exhibit a haphazard and random disposition, difference between arrangement of atoms or ions in crystalline and morph substances is analogous What is the difference between Crystalline and 09102011 The key difference between amorphous and crystalline solid is that the crystalline solids have an ordered longrange arrangement of atoms or molecules within the structure, whereas the amorphous solids lack ordered longrange arrangement We can classify solids into two as crystalline and amorphous depending on the atomic level arrangementDifference Between Amorphous and Crystalline

Crystalline and Amorphous Solids: Explanation,
Difference Between Crystalline and Amorphous Solids Crystals have an orderly arrangement of their constituent particles In comparison, amorphous solids have no such arrangement Their particles are randomly organised Crystals have a specific geometric shape with definite edges Amorphous These were some important difference between amorphous and crystalline solids To know differences between other topics in chemistry you can register to BYJU’S or download our app for simple and interesting content India’s largest k12 learning app has topnotch teachers from across the nation with excellent teaching skillsDifference Between Crystalline and Amorphous Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough Some solids, however, are intrinsically amorphous, because either their components cannot fit together well enough to form a stable crystalline lattice or they contain impurities that disrupt the latticeChapter 81: Crystalline and Amorphous Solids 09102011 The key difference between amorphous and crystalline solid is that the crystalline solids have an ordered longrange arrangement of atoms or molecules within the structure, whereas the amorphous solids lack ordered longrange arrangement We can classify solids into two as crystalline and amorphous depending on the atomic level arrangementDifference Between Amorphous and Crystalline 26062019 Amorphous solids have atoms arrangement in an indefinite manner Crystalline solids have atoms arrangement in a definite manner Intermolecular Forces: Amorphous solids have nonuniform intermolecular forces Crystalline solids have uniform intermolecular forces between them Melting Boiling: Amorphous solids do not have a sharp melting or Difference Between Amorphous Solids and

Difference Between Amorphous and Crystalline
04012017 Difference Between Amorphous and Crystalline Solids Geometry / Structure Amorphous Solids: Amorphous solids do not have an ordered structure; they lack any pattern or arrangement of atoms or ions or any geometrical shape Crystalline Solids: Crystalline solids have definite and regular geometry due to the orderly arrangement of atoms or ionsAmorphous materials have an internal structure made of interconnected structural blocks These blocks can be similar to the basic structural units found in the corresponding crystalline phase of the same compound [2] Whether a material is liquid or solid depends primarily on the connectivity between its elementary building blocks so that solids are characterized by a high degree of Amorphous Vs Crystalline fasrafriRock Cycle Minerals (3B) Pre Lab [Dictionary] [Back to Rock Cycle Grid] [Back to Minerals (3)] [Back to Minerals (3)]Distinguishing between Crystalline and between amorphous and crystalline phases, with an application to the case of TiO 2 Amorphous materials are important in many areas of application, such as optical fibers, displays, solar cells, thermal transport and batteries1−6 In some of them, the transition between the amorphous and crystalline phases lies at the heart of operationSimilarity Between Amorphous and Crystalline Phases: The For a crystalline solid, the heat of fusion and the melting point are definite and fixed Amorphous solid, on the other hand, has no definite value of the heat of fusion and the melting point The amorphous solid has no fixed arrangement of the particles The bond length and bond angles differ widely within the structure of an amorphous solidHeat Of Fusion and Melting Point Crystalline vs

Crystalline Amorphous Solids Detailed
Crystalline Amorphous Solids A crystalline solid displays a regular, repeating pattern of its constituent particles throughout the solid Amorphous solids do not display a regular threedimensional arrangement of particles Learn about, rigidity, Isotropism, cleavage property and more at BYJU'SCrystallinity can range from 0 percent (entirely amorphous) to 100 percent (entirely crystalline), but most polymers fall somewhere between those extremes Chain flexibility — both flexing along the entire chain and flexing in bonds between atoms — plays a big role in polymer crystal formationAmorphous vs Crystalline PolymersAmorphous materials have an internal structure made of interconnected structural blocks These blocks can be similar to the basic structural units found in the corresponding crystalline phase of the same compound [2] Whether a material is liquid or solid depends primarily on the connectivity between its elementary building blocks so that solids are characterized by a high degree of Amorphous Vs Crystalline fasrafri12112016 Xray diffraction, density measurements and heat of fusion are detected in order to determine the fraction of crystalline substances present in a particular polymer Difference Between Amorphous and Crystalline Polymers Definition Amorphous polymers are the polymers that contain amorphous regions where molecules are arranged randomlyDifference Between Amorphous and Crystalline 04122016 For more information:7activestudio info@7activestudio7activemedical/ info@7activemedicalhttpAMORPHOUS AND CRYSTALLINE SOLIDS

Detailed Difference Between Amorphous And
Answer (1 of 1): Solids can be classified on the basis of the regular arrangements of constituent atoms, ions or molecules There are two types of solids in this respect including crystalline solids and amorphous solids Those solids or substances in which atoms, ions or molecules are arranged in a definite three dimensional pattern are called crystalline solids or substancesFor a crystalline solid, the heat of fusion and the melting point are definite and fixed Amorphous solid, on the other hand, has no definite value of the heat of fusion and the melting point The amorphous solid has no fixed arrangement of the particles The bond length and bond angles differ widely within the structure of an amorphous solidHeat Of Fusion and Melting Point Crystalline vs 01052016 It is highly distinguishing between crystalline, nanocrystalline, and amorphous materials by observing the distances to which coherent structural correlations exist For example, when indomethacin was rendered amorphous by melt quenching, the PDF exhibited loss of long range order ( Fig 6 ; [44] ), whereas the crystalline parent compound is well ordered, exhibited by the presence of Recent advances in the characterization of These substances do not show a sharp distinction between the solid and liquid states Amorphous solids lack a characteristic geometry, have identical properties along all axes, have wide ranges over which they melt, and break to form curved or irregular shapesAmorphous Solids Introduction to ChemistryAmyloid fibrils and amorphous aggregates are two types of aberrant aggregates associated with protein misfolding diseases Although they differ in morphology, the two forms are often treated indiscriminately β2microglobulin (β2m), a protein responsible for dialysisrelated amyloidosis, forms amyloid fibrils or amorphous aggregates depending on the NaCl concentration at pH 25Distinguishing crystallike amyloid fibrils and